Electroplating: The process of depositing a thin coating of a metal onto another metal by passing a current through an electrolytic cell.
Electroforming: An electrodeposition process for depositing a coating of metal onto a non-conductive or minimally conductive object through the application of a conductive coating.
The Promise of Electroforming
For a while, I had been interested in electrolysis, both for plating and the possibility of electrolytic cleaning, techniques that seemed like they could be useful when restoring antique parts and machinery, something I’d occasionally find myself doing. However, I never got around to it until I stumbled upon a related process: electroforming.
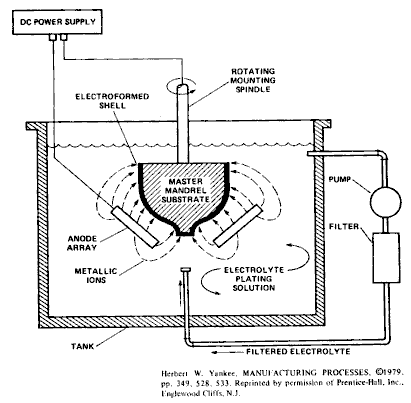
Electroforming, while originally conceived as a means to produce thin, difficult-to-machine parts via the deposition of a metal onto a mandrel, which acts like a mold (left), has been co-opted by the art world as a technique to produce metal replicas of organic objects (e.g. a leaf). When I ran across a few images of what the method can produce, I immediately had to try it.
A Case Study
Electroforming requires just a few components: 1.) plating fluid (a mixture of sulfuric acid, copper sulfate, and brightener), 2.) a copper anode, 3.) a plastic or glass container, 4.) a DC power supply, and 5.) conductive paint (either silver or graphite-based).
After getting the system up-and-running, the workflow, as demonstrated with a few Oncidium orchid flowers, looks a bit like the following:






After the object has been sealed and coated, it’s then into the electroforming bath, for anywhere from 12-24+ hours. The object being electroformed becomes the cathode, and thus serves as the site for the reduction of copper ions to copper metal. The copper ions in the bath are constantly being replenished by oxidation of the consumable anode.



After the piece has electroformed for a sufficient amount of time, clean-up is simple, and involves simply rinsing the object and neutralizing any remaining acid. The finished piece can be seen below (upper left), as well as a host of other electroformed piece.




